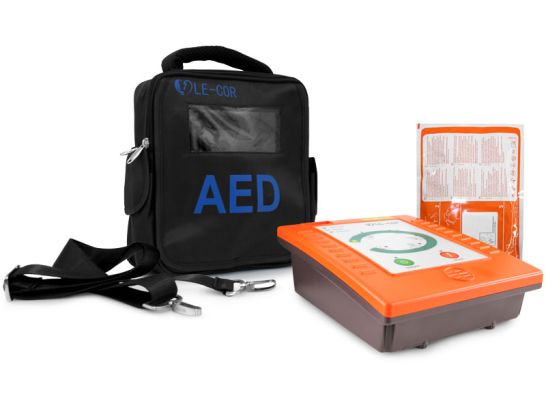
Semiautomatic external defibrillator for adults
Manufacturer Information:
Name or company: Pending update
Email: Pending update
Postal address: Pending update
City: Pending update
Country: Pending update
Responsible person information:
Name or company: Pending update
Email: Pending update
Postal address: Pending update
City: Pending update
Country: Pending update
Warnings and product safety information:
Manufacturer Information:
Name or company: Pending update
Email: Pending update
Postal address: Pending update
City: Pending update
Country: Pending update
Responsible person information:
Name or company: Pending update
Email: Pending update
Postal address: Pending update
City: Pending update
Country: Pending update
Warnings and product safety information:
Manufacturer Information:
Name or company: Pending update
Email: Pending update
Postal address: Pending update
City: Pending update
Country: Pending update
Responsible person information:
Name or company: Pending update
Email: Pending update
Postal address: Pending update
City: Pending update
Country: Pending update
Warnings and product safety information:
Manufacturer Information:
Name or company: Pending update
Email: Pending update
Postal address: Pending update
City: Pending update
Country: Pending update
Responsible person information:
Name or company: Pending update
Email: Pending update
Postal address: Pending update
City: Pending update
Country: Pending update
Warnings and product safety information:
Manufacturer Information:
Name or company: Pending update
Email: Pending update
Postal address: Pending update
City: Pending update
Country: Pending update
Responsible person information:
Name or company: Pending update
Email: Pending update
Postal address: Pending update
City: Pending update
Country: Pending update
Warnings and product safety information:
The product complies with the General Product Safety Regulation.
The AED Cor-Res A6 (DEA) is a lightweight, safe and a battery-operated device.
- It has been designed to be used in a simple and safe manner by qualified personnel.
- It can be used in cases where it can take several minutes for emergency services to arrive.
- Recognises ventricular fibrillation and other ventricular tachycardia and guides users through the defibrillation process.
- When properly connected to an unconscious patient, not breathing and with no pulse, the defibrillator analyses the heart rhythm of the patient, provides visual and voice prompts, determines whether to perform a shock and prompts the user to press the discharge button.
- Makes discharges through two low impedance adhesive and pregeled electrodes.
- Electrodes, cable and connector are sold as disposable kits.
- It is designed for INFREQUENT USE and is a DEFIBRILLATOR designed for less than 2,500 discharges.
Features:
- Advanced biphasic technology
- User-friendly: two buttons
- Compact design: easy to transfer and use
- Accessible for user: voice and visual prompts
- Safety lock system to avoid accidental activation
Cor-Res A6 is used in cases of sudden cardiac arrest (SCA), mainly caused by ventricular fibrillation (VF) or ventricular tachycardia with no pulse (VT).
Technical specifications:
1. General:
- Dimensions: 256 x 220 x 80 mm.
- Weight approx.: 1.9 kg.
- Operating temperature: 0 ºC to 40 ºC
- Operating humidity: RH between 30% and 95% (non-condensing)
- Storage temperature (with no battery): -20 ºC to 55 ºC
- Storage humidity (with no battery): up to 93 % (non-condensing)
2. Defibrillator:
- Waveform: truncated exponential biphasic
- Energy sequence: 150, 150, 200 J
- Charging time:
- 5 sec. at 150 J
- 8 sec. at 200 J
- Prompts: visual and audio
- Commands: two buttons -On/Off, Shock
- Power accuracy: ±15% for any impedance from 25 to 175 Ω
- Maximum voltage: 1050 ± 50 V
- Output banned when PATIENT impedance is out of limit: 20 Ω to 200 Ω
3. Wave specifications:
- The following table shows the details of the biphasic truncated exponential waveform obtained by Cor- Res A6 (established in 200 J) when connected to resistive loads of 25-175 Ohms.
- The waveforms are characterised by typical values for peak current (Ip), duration of the first and the second phase.
- Values are within 10% .
| Output power (J) | Patient impedance (Ω) | Ip1 (Amps) | Ip2 (Amps) | Phase1 (ms) | Phase2 (ms) | Interval (ms) |
| 150 | 25 | 46.1 | 31 | 5.6 | 3.2 | 0.7 |
| 50 | 23.9 | 15.1 | 10.7 | 6.5 | 0.7 | |
| 75 | 15.7 | 10.3 | 16.2 | 8.8 | 0.7 | |
| 100 | 11.5 | 7.7 | 19.5 | 9.5 | 0.7 | |
| 125 | 9.0 | 6.2 | 20.2 | 10.8 | 0.7 | |
| 150 | 7.4 | 5.2 | 21.4 | 10.3 | 0.7 | |
| 175 | 7.2 | 5.2 | 21.6 | 11.0 | 0.7 | |
| 200 | 25 | 53.6 | 36 | 5.5 | 3.3 | 0.7 |
| 50 | 27.8 | 17.5 | 10.9 | 6.6 | 0.7 | |
| 75 | 18.2 | 12.0 | 16.3 | 8.9 | 0.7 | |
| 100 | 13.4 | 9.0 | 19.7 | 9.6 | 0.7 | |
| 125 | 10.5 | 7.2 | 20.5 | 11.2 | 0.7 | |
| 150 | 8.6 | 6.0 | 21.6 | 10.4 | 0.7 | |
| 175 | 8.2 | 6.1 | 21.8 | 11.2 | 0.7 |
4. Electric isolation:
- Supply: The unit works only with internal battery.
- External electrical connections: No external devices connected to the unit.
- Electrical safety: Internally powered equipment with BF defibrillation -proof applied parts(according to standard IEC 60601-1)
5. Battery:
- Not rechargeable: LiMnO2 12V, 3.0 Ah
- Capacity: 100 discharges at 200 joules or 120 discharges at 150 joules
- Shelf life (25 ºC ± 15 ºC):
- 8 years (4 years storage + 4 years standby)
- 4 years standby (after installation)
NOTE: Battery capacity measured according to standard IEC 60601-2-4, clause 102.3.2 at room temperature. This capacity may decrease at extreme temperatures, or if the remaining battery is used in successives cycles on/off.
Includes:
- Unit
- Not rechargeable internal battery
- Padded backpack with several compartments and compass
- Electrodes kit for adults
- User's manual
Areas in which Cor-Res A6 (AED) can be used:
- Hospitals
- Ambulances
- Councils
- Clinics
- Health centres
- Large companies
- Sports centres
- Planes
- Shopping centres
- Cinemas
- Discos
- Gyms
- Party halls
- Amusement parks
- Airports
- Hotels
- etc...
Certification:
- Directive CE 93/42 on medical devices.