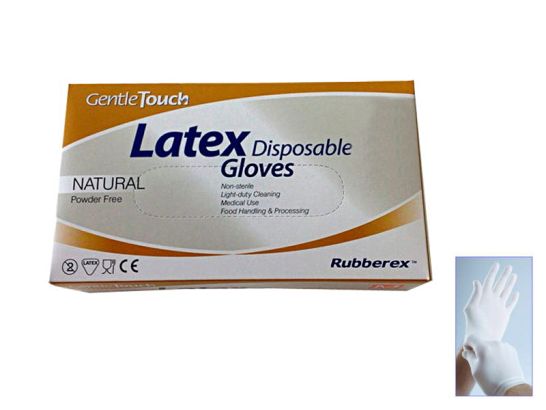
Disposable latex powder-free examination gloves Gentle Touch 100 units
Manufacturer Information:
Name or company: Pending update
Email: Pending update
Postal address: Pending update
City: Pending update
Country: Pending update
Responsible person information:
Name or company: Pending update
Email: Pending update
Postal address: Pending update
City: Pending update
Country: Pending update
Warnings and product safety information:
Manufacturer Information:
Name or company: Pending update
Email: Pending update
Postal address: Pending update
City: Pending update
Country: Pending update
Responsible person information:
Name or company: Pending update
Email: Pending update
Postal address: Pending update
City: Pending update
Country: Pending update
Warnings and product safety information:
The product complies with the General Product Safety Regulation.
Disposable latex Gentle Touch examination gloves powder free. 100 pcs. Specially manufactured to facilitate instruments handling and movement accuracy.
Features:
- 100% natural latex.
- Powder free.
- Low level of latex protein.
- Natural colour.
- Non sterile.
- Disposable.
- Specially manufactured to facilitate instruments handling and movement accuracy.
- Ambidextrous.
- Long cuff with edge for better protection against spillage.
- Anatomical shape.
- Perfect adaptability and sensitivity.
- Width: 0.30 mm -/+ 0.03.
- Elongation at break: 750%.
- Tear resistance: 20 Mpa.
- Protein content: maximum 200 ug/g.
- Length: 240 +/-0.3.
- L size = 9 size.
- Weight: 6.1 grams.
Packaging:
- Dispenser box 100 pcs with easy previously die-cut opening for an hygienic access to the glove.
- The product has been labelled in compliance with sanitary standards. It includes:
- Expiry date.
- Manufacture date.
- Lot number.
- Name and address of importing firm.
- Name and characteristics of the product.
- Brand name.
- CE mark.
- Quantity per package.
- EU standard marks: storage, warnings, disposable product, etc.
Sizes available:
- Ref. 014-LAT100PFL: L size.
Guidelines and directives:
- Real Decreto 1591/2009 that regulates medical devices according to Directive 93/42/EEC on medical devices (Class I).
- Directive 86/686 CEE- Category III.
- UNE EN 455: disposable medical gloves.
- Standard EN 455-1: warrants freedom from holes under the limit of AQL index 1.5.
- Standard EN 455-2: warrants that the gloves size comply with the European standards and meet the necessary breaking strength.
- Standard EN 455-3: warrants biocompatibility test and requirements for biocompatibility labelling.
- Standard EN 374- (1-2-3): warrants protection against microorganisms and chemical substances.
- Standard EN 420: it meets the requirements for protection gloves.
- Standard EN 388: protection against mechanical risks.
- ISO 9001:2008: quality management systems.
- ISO 13485:2003: quality management system for the manufacture of medical devices.